Is the growing number of scientific retractions necessarily due to the fruits of pseudoscience? Productive reflection on the subject leads us to the need to recognize the current demands of the scientific system for researchers, conflicts of interests at stake and that science is produced by fallible human beings with their own moral values . Globalization, while supporting misconduct, strengthens social control. Increased retraction of papers is a necessary response of the social control to what some authors refer to as “pseudoscience”. … Read More →
Ethical publishing – should plagiarized pieces be retracted ? – well, perhaps not all
An article that contains sections of texts copied from other sources (plagiarism) does not necessarily make its research bad or invalid. Even though this is a warning of unethical behavior, this does not always merit the rejection or retraction of the article concerned. This is the opinion published recently in an article in Nature. … Read More →
The SciELO journals are being improved by the adoption of classic workflows relating to the online management of article submissions
Together with the journals that it indexes, SciELO is fostering the improvement of the management of the evaluation of article submissions in order to help overcome the various difficulties faced by the journals, and to strengthen the transparency of the processes. Part of this initiative is the use of automated management systems that organize the functions of the parties involved in the evaluation of article submissions, allow for the monitoring of the workflows and provide statistics on the corresponding activities, with a view to steering the systematic improvement of the processes. This post analyzes three classic workflows in the automated management of article submissions adopted by a group of SciELO journals whose promising results show the viability of SciELO’s strategy. … Read More →
Author credits …. Credited for what?
When the publication of a scientific article has been the joint responsibility of dozens of people, the following questions can arise: Who is the author? How is credit apportioned? and Does everyone have the same level of responsibility? A recent editorial in Nature puts forward a taxonomy which can be used to categorize the different roles which come together to make up the concept of authorship. It is interesting to make a comparison with how the film industry addresses this issue when apportioning credits in the awarding of Oscars. … Read More →
The Open Data movement: international consolidation
The open data movement – the availability of scientific research data for preservation, searching, using and citing – is gaining followers in all sectors of the academic world, and with editors, publishers, research institutions and funding agencies. The movement will allow greater interoperability, transparency, visibility and research impact, in addition to ensuring the digital preservation of the original data that would otherwise have a tendency to be lost or become inaccessible with the passage of time. … Read More →
South America science in Nature
The celebrated journal Nature devotes a special section of its June 11 issue to an analysis of South American research output, highlighting areas of excellence and innovation which are internationally recognized, and fields of collaboration with other countries both inside and outside the region. The articles in this section stress the economic and infrastructural inequalities within the region, as well as the low levels of investment in research and development, and point to FAPESP as a successful model of investment directly linked to a state’s GDP. … Read More →
The challenges in professionalization
The professionalization of the management and operation of journals indexed by SciELO is facing challenges related to SciELO’s central position in the academic environment, with particular reference, on the one hand, to the rational use of the limited time that researchers dedicate to the position of Editor-in-Chief and, on the other hand, to the adoption of editing, publishing, dissemination and marketing services in line with the international state of the art. … Read More →
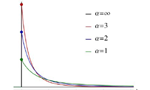
What is the decline of the elite journals?
According to a study by Vincent Larivière of the Université de Montréal, there was an exponential increase in the number of articles published in the elite journals. However, it is now required twice the number of citations than in previous decades for an article to be in the top 5% with higher impact, and that these articles are distributed amongst a wider base of titles because authors have more independence in choosing where they publish. … Read More →
Ethical Publishing – the time line of a case of plagiarism
The most advanced research, which is published in elite journals such as Nature, is not beyond plagiarism or serious misconduct. In a recent case in stem cell research at Weseda University in Japan, the university ordered a review of the 280 theses produced at the RIKEN Institute since its founding in 2007. … Read More →
Non-native English-speaking authors and editors evaluate difficulties and challenges in publishing in international journals
Due to linguistic and cultural barriers, authors in emerging economies have faced challenges in having their papers accepted in main stream journals. A study conducted on international editors and authors in non-English speaking countries shows that good research results can be prejudiced by poor writing and difficulties with the language. … Read More →
Scientometrics of peer-reviewers – will they be finally recognized?
ORCID and CASRAI have initiated a project to establish a set of standard procedures to recognize the work of peer-reviewers. In this way the important work of peer-review, almost always anonymous, will be counted in the acknowledgments and be incorporated into scientometric indicators and altmetrics. But this will depend upon a sufficient level of adoption and acceptance of the procedures. … Read More →
SciELO Brazil revises indexing criteria
The indexing criteria that guide the journal review process to determine the inclusion and permanence in SciELO Brazil is being updated. The new criteria will be announced in the second half of 2014. The main objective is that indexing criteria reflects the priority lines of action of the SciELO Program to increase the visibility and impact of the research it communicates. … Read More →
The EU will centralize the registry of clinical trials
In April 2014, the European Union approved legislation regulating the registry of clinical trials. This will allow for transnational cooperation between laboratories and institutions of research. The measure will contribute to the transparency and dependability of the trials, and will also allow research into drugs for the treatment of rare diseases. The first registry of clinical trials was created by the WHO in 2004. Currently, registering clinical trials is mandatory in the majority of the countries. … Read More →
Ethical publishing – scholars have to make bibliographical references as well
Should top-flight scholars include bibliographic references in their works to sources they have used or is it the case that the bibliographical reference is an archaic technicality? This question became a topic for discussion at the beginning of this month because of the accusations made against Zygmunt Bauman, namely that his latest book includes sections of text copied from web sites and Wikipedia – a procedure known as “mosaic plagiarism”. … Read More →
Study highlights academic journal publication models in Brazil and Spain
Brazil and Spain have a commanding profile in academic output in their respective regions, and possess great potential in the field of scholarly publishing. Notwithstanding the differences in the history of scientific development between the two countries, both have a similar number of journals in the Web of Science database and have developed successful open access models. This article highlights a study carried out by researchers from those two countries and gives an outline of these programs and the reasons behind their success. … Read More →























Recent Comments